Chemical Reactions in Gas Phase
Hydrogen gas and oxygen gas react to produce water vapor. The hydrogen gas and the oxygen gas react at a volume ratio of 2: 1 to generate 2 volumes of water vapor.
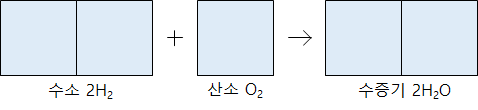
When the gas reacts at a constant temperature and pressure and a new gas is produced, there is always a simple integer ratio between each gas’s volumes.
The volume ratio is expressed as a simple integer ratio because the number of molecules in the same gas volume is the same at the same temperature and pressure regardless of the gas type. In 1811, Avogadro, an Italian scientist, published it as a rule.
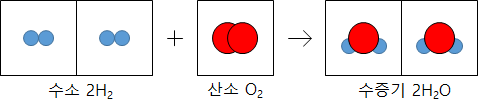
The coefficients ratio is the same as the number of molecules in the chemical reaction formula. The gas reacts, and the ratio of the number of molecules is equal to the volume ratio.