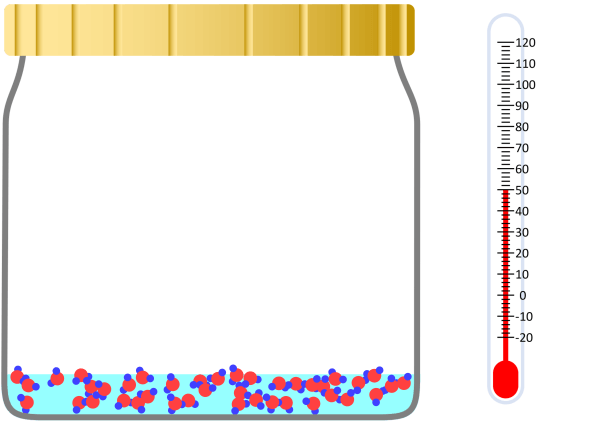
Boiling Point (Ice or Water, Ethanol)
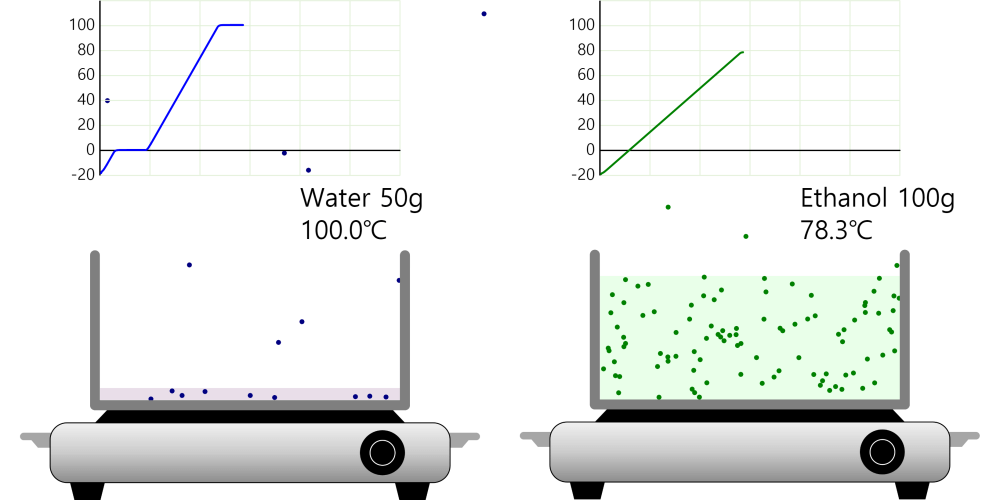
Boiling point and properties of substances All water boils at 100°C when heated, even if it has a different mass. In this way, the same kind of substance has the same boiling point regardless of quantity. But different substances express … more