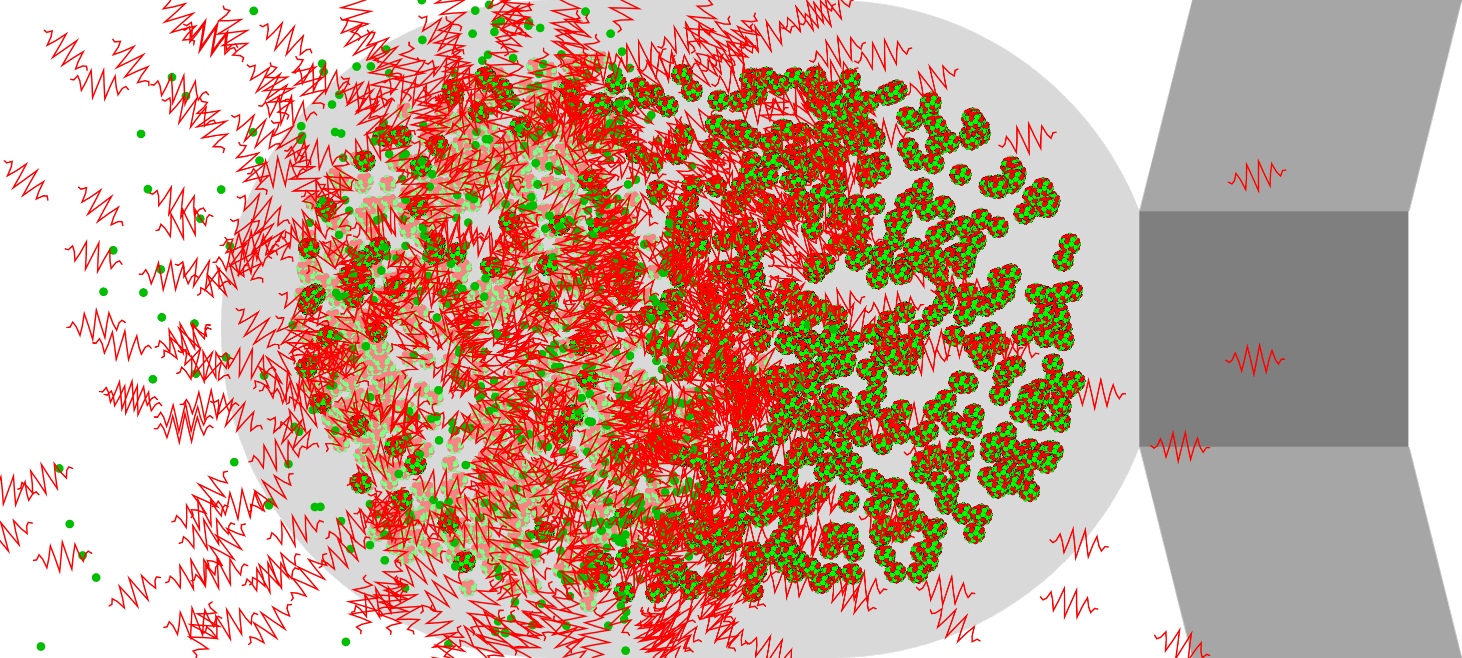
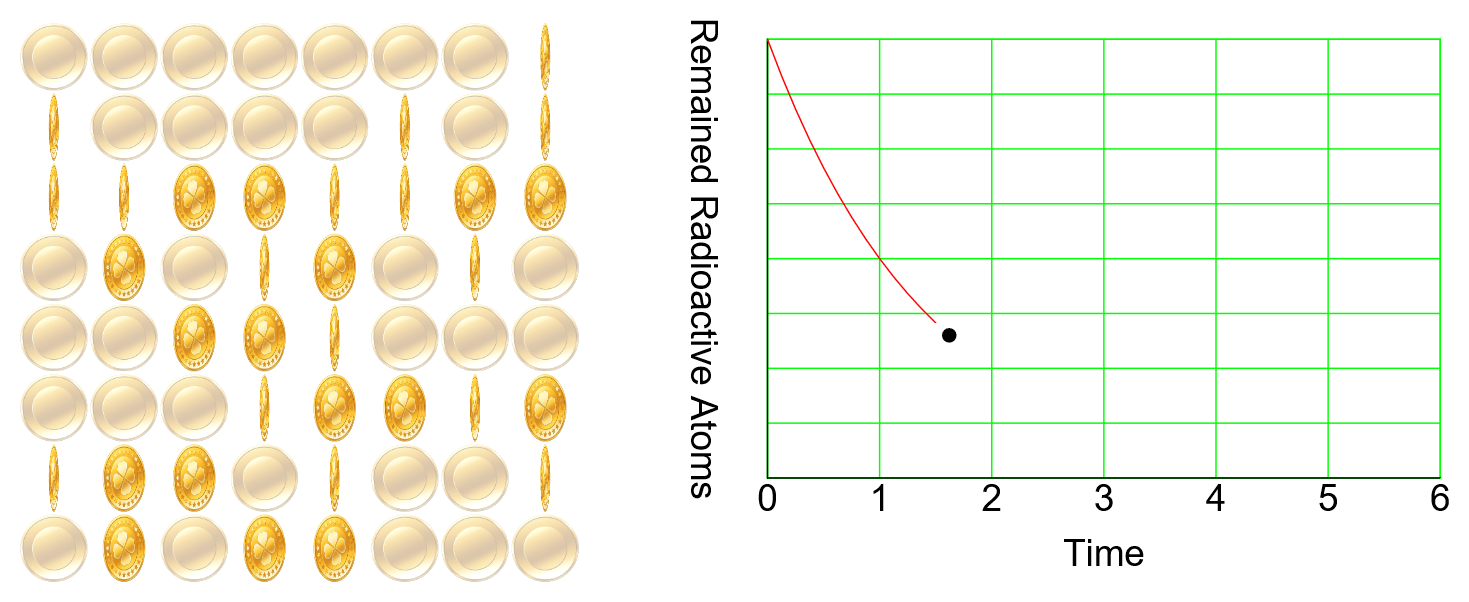
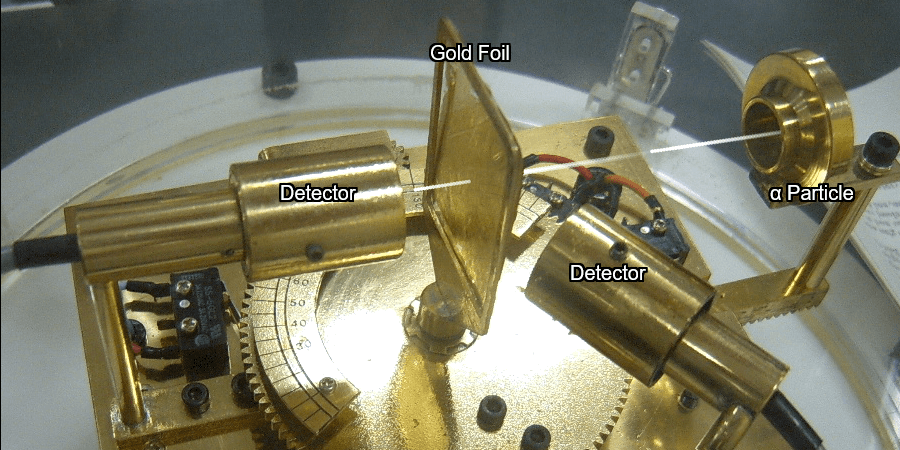
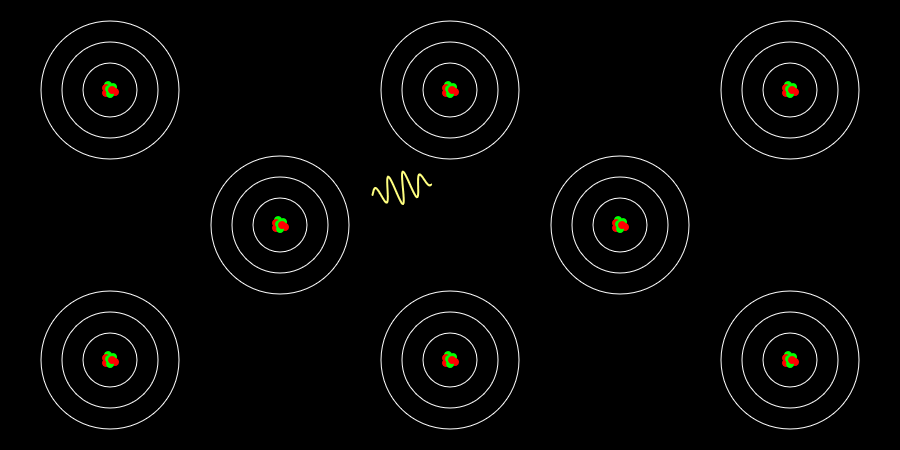
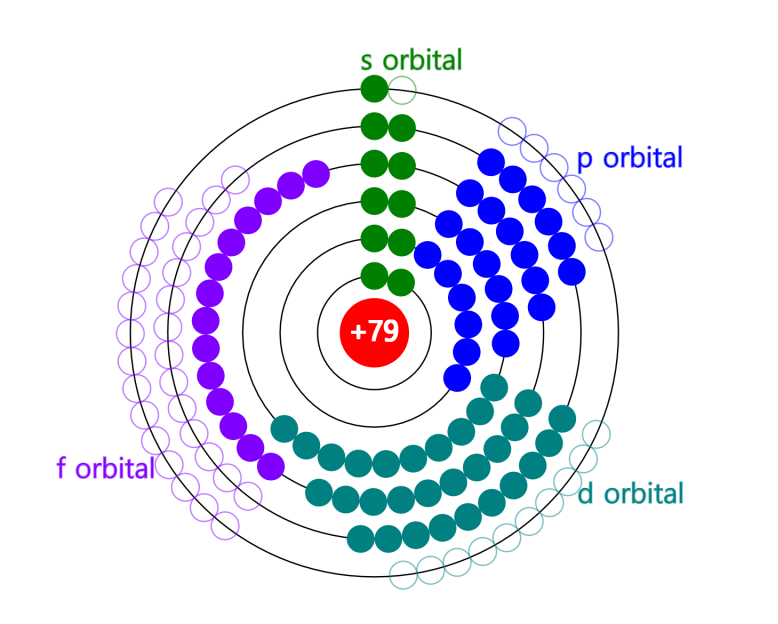
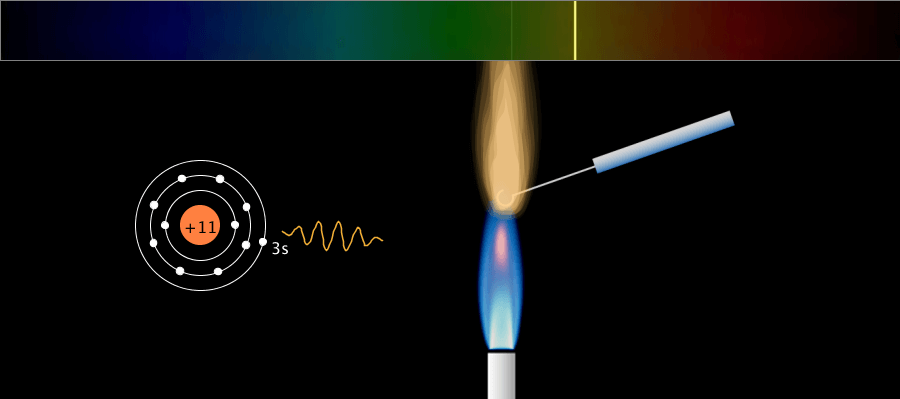Nuclear Chain Reaction
Caution This simulation is intended to understand the principle of fission, and the proportions of the model presented may not match the reality. The nucleus was exaggerated and drawed large. The electrons around the nucleus were … more