Energy band and band gap of atom
According to Niels Bohr's atomic model, electrons subordinate to an atomic nucleus do not have continuous energy. Electrons can only have sparsely defined energies. This is why lines in the atomic model represent the orbits of electrons.
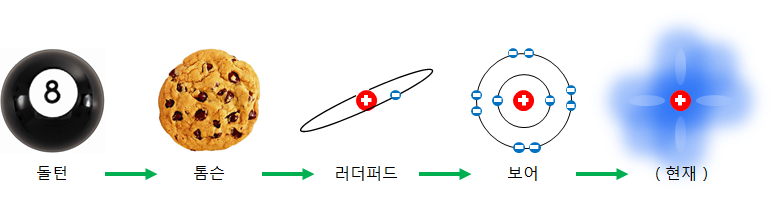
If there is only one nucleus alone, this explanation will end, but if the nuclei are densely attached like a solid, the atoms' electron orbits overlap each other and become thicker.
As a result, the energy level now takes a band (band) with a certain width rather than a single line.
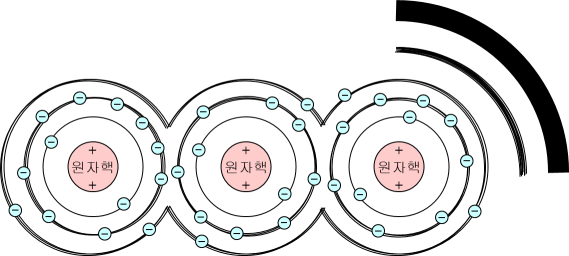
This thickened energy level is called an energy band.
And there is a bandgap Between the bands that electrons cannot exist.
The importance of energy bands
The energy band is a very important concept in metal and semiconductor engineering.
Metals become conductors that conduct electricity well because electrons can move freely around them through energy bands. Many nonmetal's electrons are difficult to move because electrons fill out the energy band.
Semiconductor
If you use the energy band and the bandgap well, you can control the current flow according to the surrounding physical situation. This property can also be used instead of a physical switch. The remarkable advancement of computers and other electronic devices is the utilize of this property.