Photoelectric Effect
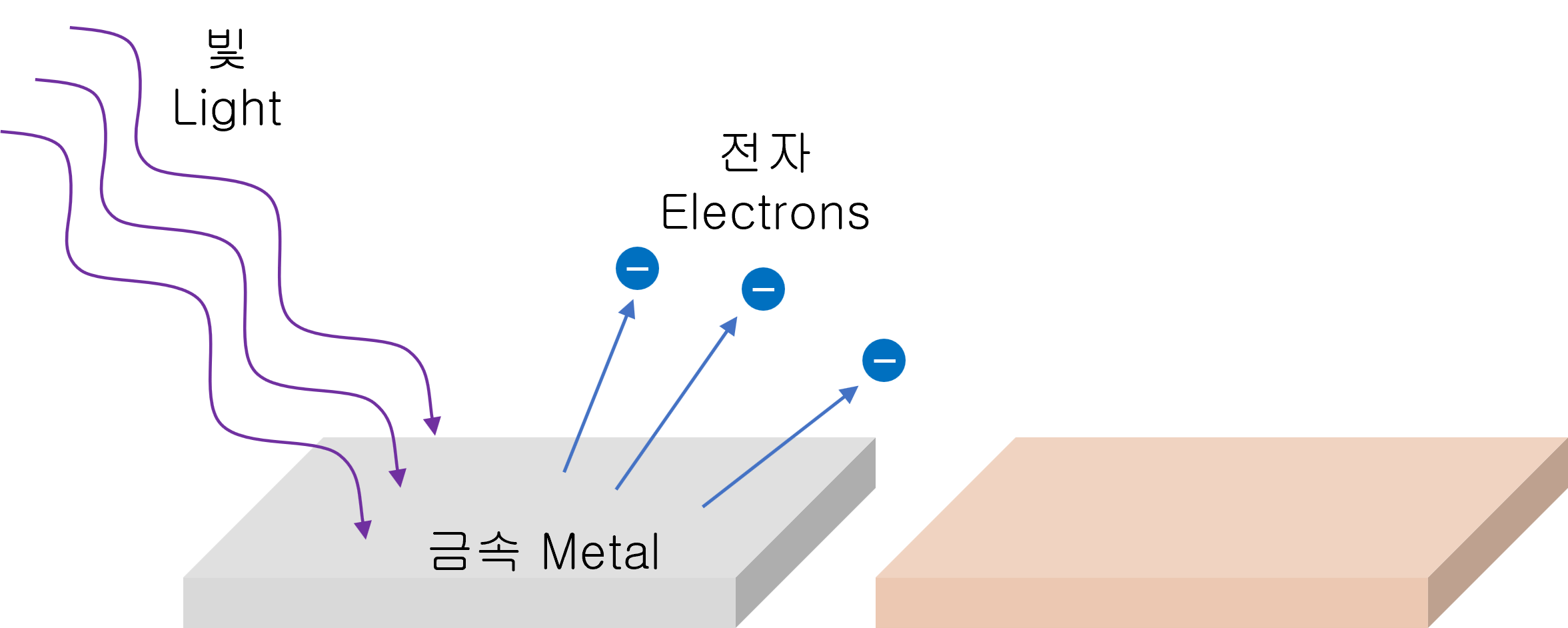
The photoelectric effect is the phenomenon that the electrons pop out when a light beam incident on a metal surface.
It can be thought that the energy of light is transformed into the form of electrical energy.
However, electrons will only be emitted when the light is incident on the metal at a certain frequency or higher. The minimum photon energy at which electrons can stick out is the 'work function.'
Usually, alkali metals such as sodium (Na) are often used in photoelectric effect experiments. This is because alkali metals' work function is small, making it easy to cause a photoelectric effect even with low-frequency visible light.
Future energy source, the sun
This photoelectric effect becomes the basic concept of solar power generation. In other words, when light is emitted above a certain frequency, electrons pop out immediately (no delay in time).
If these electrons are allowed to run through electrical circuits, then solar power is generated.
Solar cells are semiconductors made from silicon (Si) in the sand. When sunlight shines on a solar cell, the electrons move and flow along the wires, and the flow of electrons creates electrical energy.

Solar cells are made by adding tiny amounts of phosphorus and boron to each side of thin silicon plates, which are commonly used as semiconductors.
Most commercially available silicon solar cells can convert 15 to 25 percent of the energy in sunlight.
The output voltage of one solar panel is only about 0.6V. So we connect several plates to increase the output voltage and current.