Boyle’s J-tube experiment
Boyle’s J-tube experiment is an experiment that Boyle did to prove his law. Boyle experimented with pouring mercury into a J-shaped glass tube with one side blocked.
At first, the heights of mercury are equal to each other. Because the open side is facing the outside air, it is 1 atm. Since the mercury heights are the same, the clogged air’s atmospheric pressure is also 1 atm.
Boyle poured mercury into the open side glass tube. The height of the mercury in the open side glass tube was increased, and the volume of the clogged side glass tube was reduced. Boyle calculated the mercury pressure from the height difference. And Boyle measured the reduced volume of the clogged glass tube.
From this experiment, Boyle found that ‘at constant temperature, the volume of the gas is inversely proportional to the pressure.’ This relationship is called Boyle’s law.
Analysis of Boyle’s J-tube experiment
Initially, the air pressure on both sides is the same at 1 atm (760 mmHg), because the mercury is at the same height on both sides.
When the mercury is poured, a height difference occurs. This difference is due to the different air pressures on both sides.
Calculating the pressures individually is as follows:
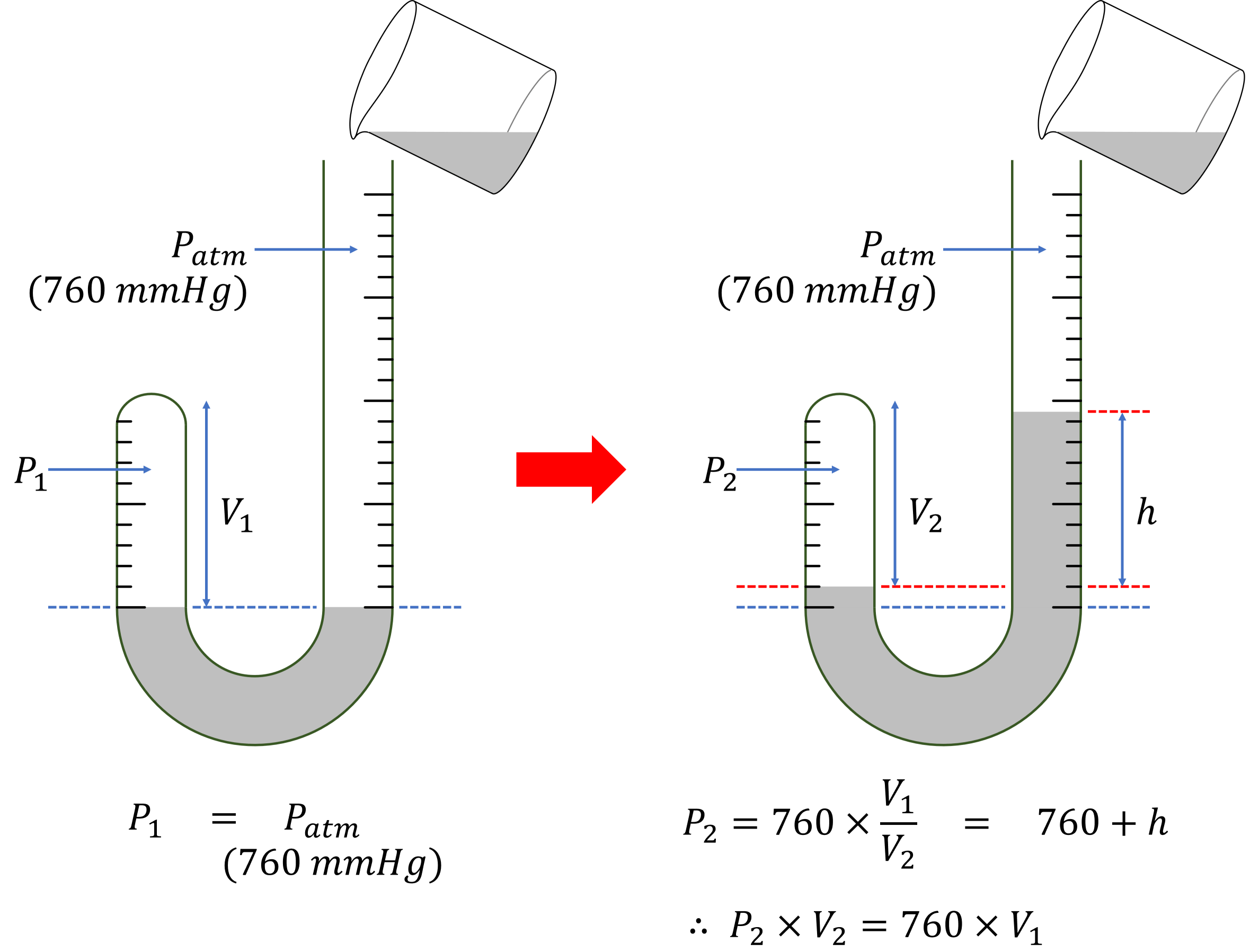
Here, \(760\) is the pressure unit of a mercury column (mmHg), equivalent to 1 atmosphere.
In a closed system, \(P_2 \times V_2\) is the product of pressure and volume, which is equal to \(760 \times V_1\). Since \(760\) and \(V_1\) are constants, \(P_2 \times V_2\) is also constant.