Avogadro's Law
The Italian scientist Avogadro (1776-1856) introduced the molecules' concept to explain gases' reactions. According to his theory, the volume of any gas at the same temperature and pressure is proportional to the molecules' number.
In other words, regardless of the type of gas, the number of molecules is the same in the same volume, same temperature, and same pressure.
Avogadro's molecular theory
Avogadro knew that the atomic model could not explain that gases' reaction volume ratio is established as an integer ratio. To solve this, the concept of a molecule was first proposed. The reaction of hydrogen and oxygen to form water vapor is expressed in a molecular model as follows.
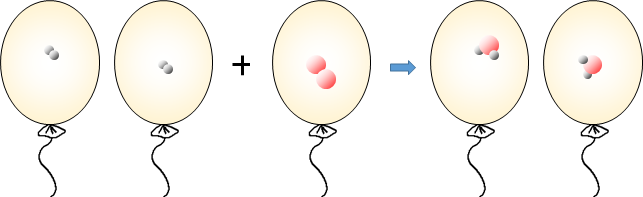
In this way, when a gas reaction is expressed in a molecular model, the type and number of atoms do not change before and after the reaction. A constant integer ratio is established between the gas volumes, and the atoms do not split. Therefore, the molecular model can well explain the volume ratio that occurs when gases react. Based on this explanation, Avogadro came to think that gases exist not as atoms but as molecules. As can be seen from the molecular model, a molecule is a particle made up of atoms bonded together and is the smallest particle with the character of matter.