The discovery process of the atom
- Democritus (Ancient Greece) – Predict the presence of an atom
- Dalton (UK, 1800) – Applied atomic chemistry
- Krux (Britain, late 19th century) – Invented a cathode ray tube to explain various phenomena of electrons
- Rutherford (Great Britain, 1909) – Confirming the existence of atomic nuclei
- J. J. Thomson (UK, 1897) – Determination of the presence of electrons through a cathode ray tube experiment
- N. Bohr (Denmark, 1913) – Planetary hypothesis of the atom
- A. N. Bohr (Denmark, 1922) – Describing the shape of an atomic nucleus
- de Broglie (France, 1923) – Propose the oscillation of electron particles
- Schrödinger (Austria, 1926) – Mathematically identifying the electron orbits of an atom
- Heisenberg (Germany, 1927) – Describes the stability of the electron orbit through the uncertainty principle
- R. Feynman (USA, 1965) – Quantum Mechanics Research
- IBM (USA, 1990) – Making a microscope capable of viewing atoms, succeeding in moving atoms into particles
Atomic model
What is the shape of the atom? The atomic model is a way of explaining what an atom looks like. The atomic model has been transformed as follows.
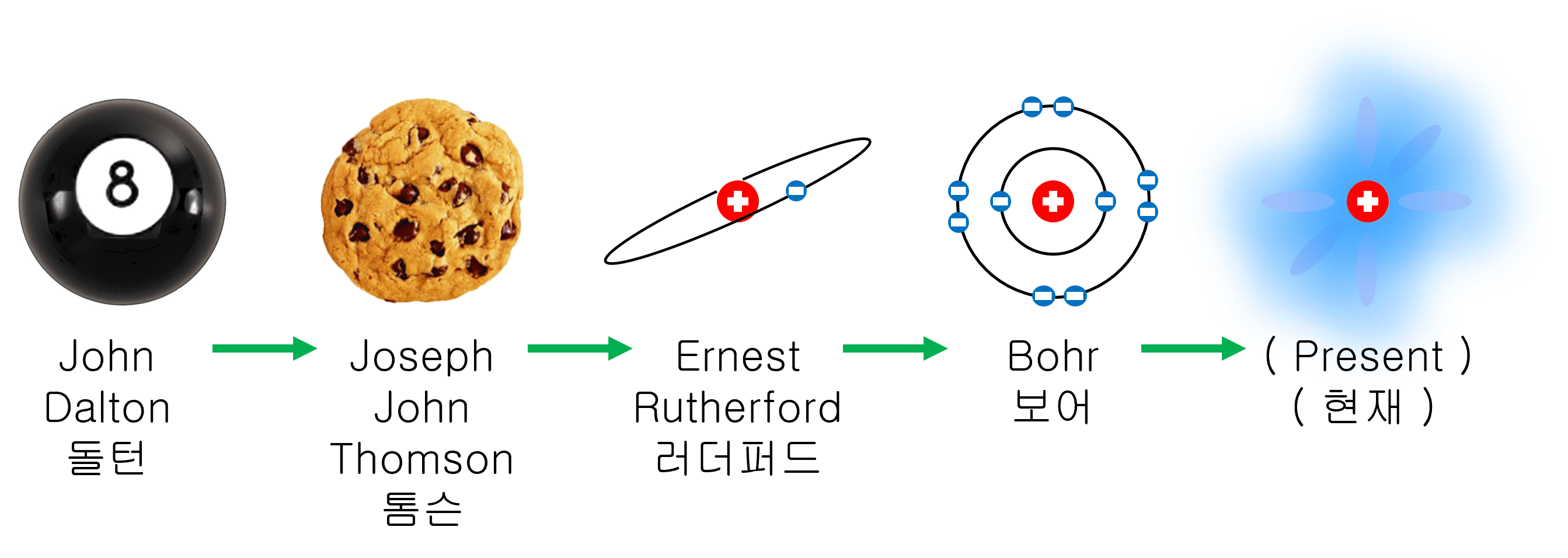
When We didn’t know much about the atom, we thought it would be a solid lump like a billiard ball. However, real atoms can be divide into electrons and atomic nuclei, and using a particle accelerator can also divide atomic nuclei.
About 200 years ago, an atom was imagined to be like a muffin with raisins. The raisins on the outside of a muffin can be compared to the electron. Positive charges within an atom are spread throughout the entire volume, and electrons were thought to oscillate around fixed points within a cation (muffin).
One hundred years ago, it was discovered by Rutherford that most of the atoms were empty.
After that, Niels Bohr presented a more refined atomic model. Bohr’s atomic model is very close to the atomic model that can be seen these days. In Bohr’s atomic model, electrons are in a given orbit. This model is also called a planetary model because electrons orbiting around an atomic nucleus resemble a planet orbiting the sun.
Currently, it is known that electrons around an atomic nucleus have the properties of matter and wave at the same time, and the position and speed of electrons cannot be accurately known at the same time and can only be grasped with statistical probability.