Discovery of nuclei
At the beginning of the 20th century, little was known about atoms’ structure, except that atoms contain electrons.
At the time, the atom was thought to be as a model of pudding containing raisins. According to this model, positive charges in an atom are evenly spread throughout the entire volume, and electrons (raisins) are thought to oscillate around fixed points in a sphere of charge (pudding).

In 1911, Rutherford experimented with colliding powerful α-particles (the nuclei of helium) into thin pieces of gold foil.
Α-particles that are 7300 times heavier than electrons have a charge of +2e and are naturally released with the energy of several MeVs from many radioactive materials.
With this experiment, Rutherford attempted to measure how much α-particles bend as they pass through a piece of gold leaf.
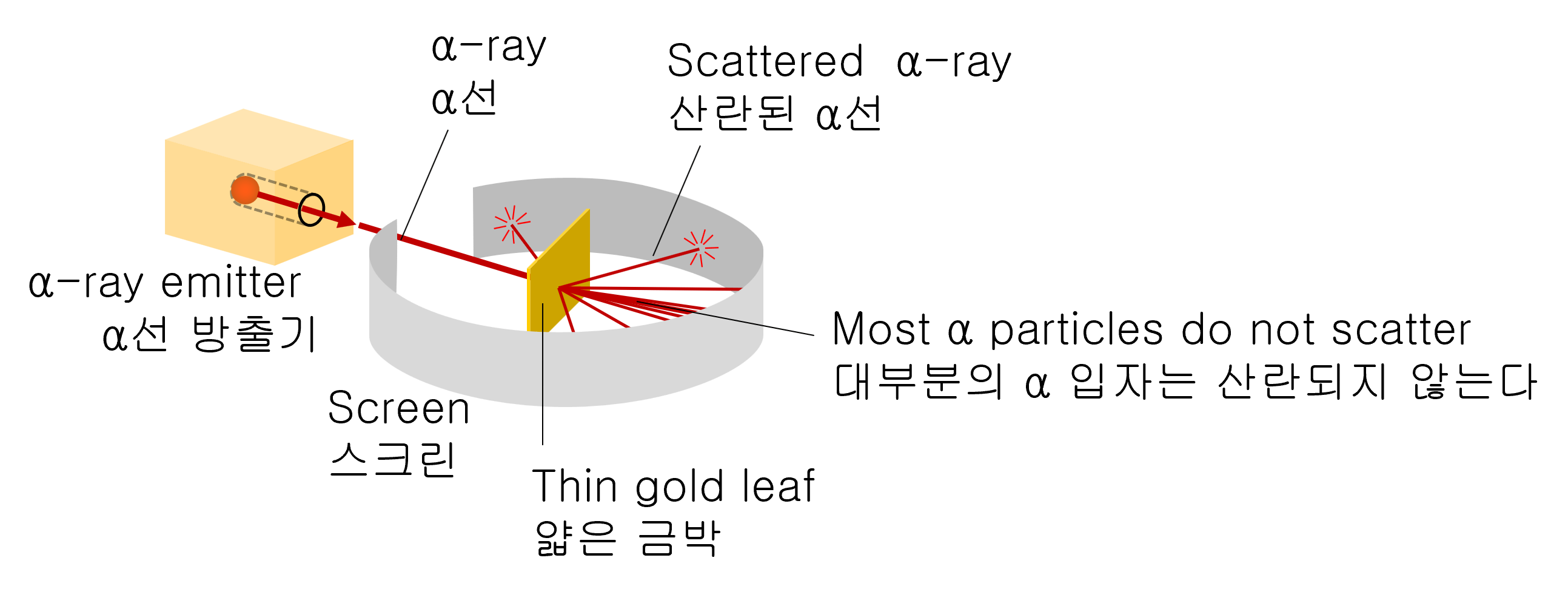
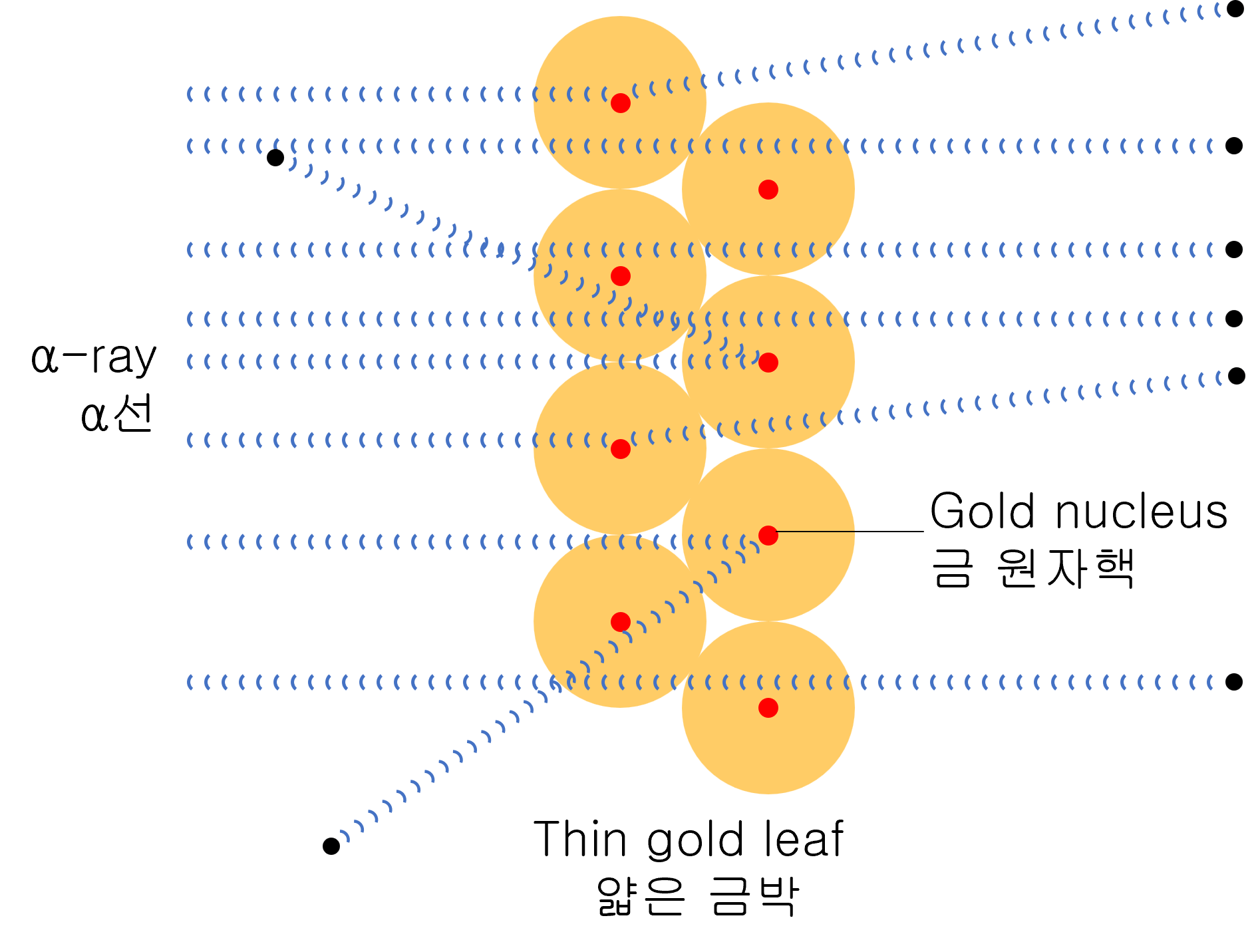
As a result of the experiment, most α-particles are scattered at small angles, but surprisingly, tiny amounts of α-particles are scattered at large angles close to 180 degrees.
Through this experiment, humankind became aware of the existence of an atomic nucleus.
Analyzing the results of the experiment, Rutherford came to the following conclusion:
“The radius of the nucleus must be much smaller than that of the atom, and the ratio is about 104. In other words, most of the atoms are made up of empty spaces.”
It is not uncommon to draw such important conclusions from a brilliant scientist’s keen insights and a few simple calculations.

An experimental device used to test the scattering of α (alpha) particles by a thin piece of gold foil. The detector can be rotated with several scattering angles. The atomic nucleus was discovered with such a simple experimental device.