The ductility and malleability of metal
When a force is applied to a metal crystal, the metal atom layer is pushed, and the atomic arrangement changes. But at this time, the free electrons move and maintain the bond between the free electron and the metal atom. Thus, the slipped layer does not fall off, and the atomic arrangement's regularity does not change.
Features of metal bonding
If you hit the soft metal (ex. lead) with a hammer, it spreads thinly, but if you hit the salt crystals, it breaks. Why?
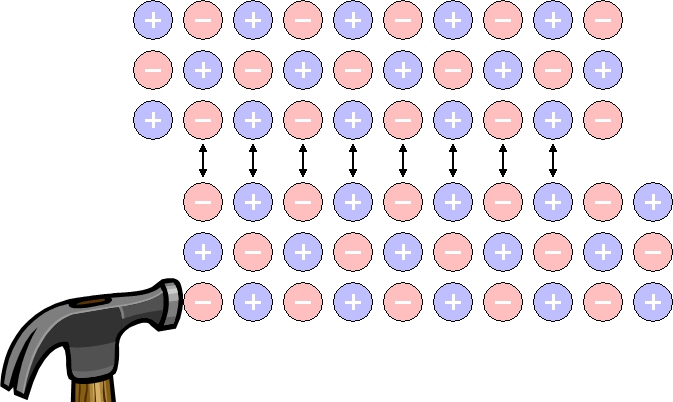
Ionic bond crystals don't stretch, they just crumble.
Unlike metal crystals, salt is a material in which cations (Na+) and anions (Cl-) are tightly bound at a fixed position. Therefore, when a salt crystal is hit with a hammer, an overlapping layer of cations and cations and anions and anions is formed and repels each other, making them brittle. However, metal crystals do not break because electrons move and hold bonds between metal atoms.