Ionization tendency of metal
The tendency of metals to lose electrons and become cations is called the ‘ionization tendency.’ The ionization sequence is an ordered list of ionization trends.
The greater the metal’s ionization tendency, the easier it is to oxidize and the more reactive it is.
Conversely, metals with a low ionization tendency are more likely to be reduced. In other words, it tries to exist as metal.
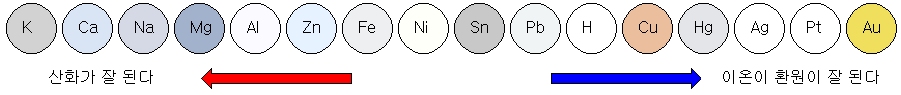
Comparison of reactivity of metals
The reactivity of metals can be compared to each other, depending on whether a metal is reacted in another metal ion’s solution.
If the reaction occurs when a metal is put into an aqueous solution of another metal ion, the reactivity of the inserted metal is higher than the metal in the solution.
If the reaction does not occur when the metal is put into an aqueous solution of another metal ion, the reactivity of the inserted metal is lower than the metal in the solution.